Sunday, May 29, 2011
Chemistry Composting Part 2
Oxygen is an important factor to consider when composting.
Aerobic Composting: An aerobic process in the decomposition of organic materials in the presence of oxygen. Composting can be an aerobic process. As compost is consumed, the carbon to nitrogen ratio goes from 30:1 to 10-15:1. This is because two thirds of the carbon is released as carbon dioxide gas. The carbon is oxidized and carbon dioxide is produced. The remaining third is combined with nitrogen in living cells. The oxidation of carbon is an exothermic process. For every gram of glucose 484-674 kcal of heat are produced. This means that compost heaps can have temperatures above 170 degrees Fahrenheit. The atmosphere is twenty-one percent oxygen, but a compost heap only needs to maintain an oxygen percentage of ten percent to be aerobic. Some compost mixtures naturally maintain healthy oxygen levels by diffusion and convection, while other require active aeration. Blowers or periodic turning and mixing can aerate composts. Compost that is not aerated will become anaerobic. Aerobic composting takes place in bins, pits, or stacking piles.
Anaerobic Composting: Another type of composting is anaerobic- the decomposition of organic matter without the presence of oxygen. Unlike aerobic composting, anaerobic composting is a reduction process. Carbon is reduced to methane gas. Hydrogen sulfide gas is another byproduct of anaerobic composting.
As a result anaerobic composting releases pungent odors. The final product in the reduction process is humus. Some minor aerobic oxidation takes place at the end, but it is negligible. Therefore, anaerobic oxidation releases significantly less heat than aerobic. As a result, pathogens are not destroyed by heat but disappear naturally due to unfavorable condition- a much slower process. Nitrogen is reduced to organic acids and ammonia. Anaerobic compost must sit for up to six months to a year to insure pathogens are no longer present. Anaerobic composting takes place in large well packed stacks. It requires much more water than aerobic so that oxygen cannot penetrate the mixture. When the mixture is eighty to one hundred percent saturated, the organic material becomes suspended in a liquid.
Whether the gardener chooses aerobic or anaerobic composting, there are some basic dos and don’ts of composting. A good balance of green and brown materials will lead to an ideal balance of nitrogen and carbon. However do not include any meats, cooked vegetables, dairy, animal waste, perennial weeds or salad heads.
The Fertilizers in our Gardens
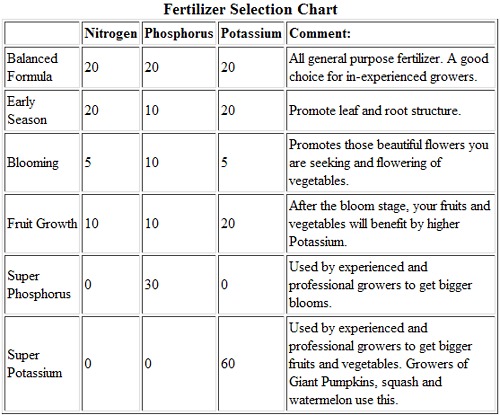
Another way chemistry is connected to gardens is through fertilizers. Fertilizers are commonly regarded as materials that are added to the soil or plant to supply one or more elements that are essential for plant growth. Most people are unaware that fertilizers are chemicals, so treat them with care and use protective gloves. Make sure to avoid inhaling odors. The standard fertilizer will have a label reading “20-20-20”, which means that that particular fertilizer has 20% Nitrogen by weight, 20% Phosphorous by weight, and 20% Potassium by weight.
Higher concentrations of Nitrogen should be applied in the early growth stage, as it contributes to leaf root growth which results in a lush, green plant. However, too much Nitrogen can reduce or delay the growth of flowers and fruit, so if your plant is a healthy green, seems to growing well, but doesn’t have flowers, stop adding Nitrogen. You should start using fertilizers that have a higher concentration of Phosphorous when the season moves towards the flowering and fruit set stage. Phosphorous promotes flower growth, root growth, and fruit set and development. Potassium promotes fruit growth. After fruit set, you should start using a high potassium fertilizer. Below is a chart demonstrating possible fertilizer variations.
As mentioned above, there are nitrogen, phosphorous, and potassium fertilizers. There are also quick release/slow release fertilizers, and stabilized nitrogen fertilizers. Anhydrous ammonia is the main component in nitrogen fertilizers, and is produced by the Haber-Bosch process:
3H2+ N2 + heat, pressure, & catalyst = 2 NH3(g) (anhydrous ammonia)
Anhydrous ammonia (82-0-0) is sold as a liquid under pressure and when injected into the soil, undergoes the following reaction:
NH3(g) + H2O <— —> NH4OH <— —> NH4 + OH- —> NO3
The raw material in phosphorous fertilizers is rock phosphate or apatite. They create a superphosphate through the following reactions.
- Apatite + sulfuric acid —> superphosphate.
- Apatite + phosphoric acid triple —> superphosphate
Quick release fertilizers are readily available and are very water soluble. Slow release fertilizers, on the other hand, are less soluble due to the fact that a coating is added to the fertilizer in the granule that delays the rate of dissolution. The advantages of slow release fertilizers include: lower potential to cause injury to plants, higher Nitrogen use efficiency by plants, less subject to volatilization. One disadvantage of slow release fertilizers is that they are fairly expensive. Stabilized nitrogen fertilizers are products mixed with or added to Nitrogen fertilizer materials that either act as a urease inhibitor or a nitrification inhibitor. The fertilizer is said to be stabilized because it is not as subject to losses from volatilization or leaching. This fertilizer is used widely in corn producing areas of the U.S. It is a liquid that is mixed with anhydrous ammonia. In places where the microbial reactions in the soil are already slowed down (perhaps due to cool soil temperatures, as in Alaska) the use of this fertilizer may reduce Nitrogen availability to crops.)
Chemistry Composting
In researching the links between chemistry and gardening, composting arose as a key component. While composting merely entails the accumulation of waste outdoors, there is a surprising amount of chemistry involved.
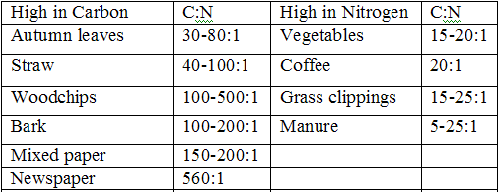
The decomposition of organic materials is rich in nutrients that promote plant growth. When organisms die their stored carbon and nitrogen becomes available to other organisms. Any compost will have a balance between bacteria and plant materials. This is to maintain a healthy carbon to nitrogen ratio. Bacteria typically have a ratio of 4:1, while plant material has 30:1. Carbon and nitrogen are both important elements in plant growth. Carbon is an essential energy source and a building block for plants, as it composes fifty percent of microbial cells. Nitrogen is an important component of proteins, nucleic acids, amino acids, and enzymes. While carbon and nitrogen are both essential, neither is useful independent of the other. Diamonds would be detrimental to a compost heap. The ideal carbon to nitrogen ratio for composting is thirty to one. An excess of nitrogen will cause it to form ammonium gas, toxic to plants, and releasing pungent odors. Conversely, a compost heap deficient in nitrogen will restrict plant growth.
Below is a table of a variety of different plants and their carbon to nitrogen ratios.
Friday, May 27, 2011
Soil and its pH

We continued to discover the connections between chemistry and gardens, and found out quite a lot about soil pH. At first, we didn’t think there’d be a way to take the pH of the soil, or if it was even important. After doing some research, we discovered that there are several different ways to take the pH of the soil, some of which are listed below.
- Use an inexpensive pH testing kit based on barium sulphate in powdered form. When a small sample of soil is mixed with water, the color will change according to the acidity or alkalinity.
- Use litmus paper. A small sample of soil is mixed with distilled water, into which a strip of litmus paper is inserted. If the soil is acidic the paper turns red, if alkaline, blue.
- Use a commercially available electronic pH meter, in which a rod is inserted into moistened soil and measures the concentration of hydrogen ions.
- pH 4.5-5.0: blueberry, cranberry, orchid, blue hydrangea
- pH 5.0-5.5: parsley, potato, radish, ferns, sweet potato
- 5.5-6.0: beans, carrot, brussels sprout, peanut
- 6.0-6.5: broccoli, cauliflower, cabbage, cucumber, tomato, strawberry
- 6.5-7.0: asparagus, beet, celery, lettuce, spinach, onion
- pH 7.1 - 8.0 lilac, brassica
Be sure to check back at our blog for more information on the incorporation of chemistry in gardens!
Monday, May 23, 2011
The Beginning!
Santa Monica!
We are students in AP Chemistry at Santa Monica High School. Our class has decided to take on a service learning project. In this month and next we will be turning gardening into a community effort. Our goal is to sustain a school/community garden at Santa Monica High School that can effectively make an impact on education and the school. In addition, we will incorporate Chemistry into helping the garden and growing produce.
Please follow our blog, facebook (www.facebook.com/samohigarden), twitter (www.twitter.com/samohigarden), and Tumblr (www.samohigarden.tumblr.com)!
Thanks for the support and check back soon,
The Samohi Garden
Subscribe to:
Posts (Atom)